What it is and how it works?
Spermidine... ok so it's not got the best marketing name on the planet (it's a polyamine found in many foods but was originally isolated in semen, hence the unfortunate name) but it's certainly one of the hottest longevity and anti-aging molecules on the block.
One you need on your radar if you are interested in slow the hands of time.
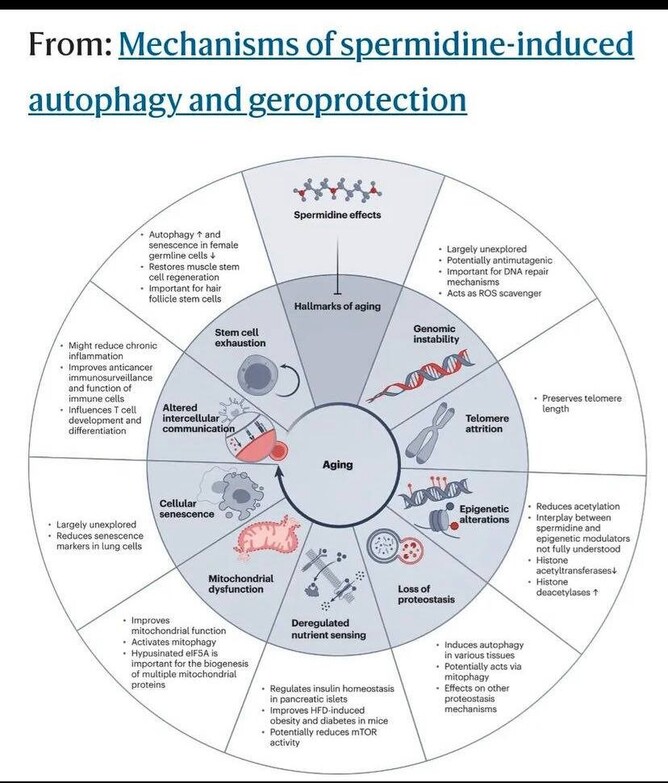
Autophagy is the main mechanism spermidine induces at the molecular level, but evidence has been found for other mechanisms, including inflammation reduction, lipid metabolism, and regulation of cell growth, proliferation, and death.
So what is Spermidine?
Spermidine is a polyamine derived from putrescine (another fabulous name) that is involved in many biological processes, including the regulation of membrane potential, the inhibition of nitric oxide synthase (NOS) and the induction of autophagy.
It is a precursor of spermine.
Spermidine has been the subject of 30+ clinical studies and trials with hundreds of research groups around the world currently doing continuing research.
Spermidine is a unique, naturally occurring molecule, part of a group of molecules known as polyamines. Polyamines interact with our cells to perform various critical metabolic functions. Spermidine is found in all plants and animals and in nearly every cell of your body. In our bodies, it can come from ingested food—synthesized in the gut microbiome or synthesized in cells.
As we age the levels of Spermidine decline.
Levels start declining as early as 25 years old. But one study done to 90 to 100+ year olds living in the so called "Blue Zones" showed that these people had the spermidine levels of young people. Is this one of the secrets to their long lifespans?
Where can you find Spermidine?
Spermidine is a natural compound found in many foods such as green peppers, wheat, broccoli, mushrooms, and many cheeses. It is a polyamine molecule which plays an important role in cell metabolism and small amounts can be found in all eukaryotic cells.
What is Autophagy and why is it important?
Autophagy is a Greek-derived term that means "self-" (auto) "eating" (phagy). it's actually your body's way of clearing out old, damaged cells and proteins to make room for new, healthy ones. Autophagy is the ultimate natural body cleanse.
Clearing out old and damaged cells is essential. Damaged cells are called Senescent cells
Warning... incoming sciency explanation but bear with.
A senescent cell is one whose life cycle has come to a permanent end. Senescence is a cause of certain diseases, as we’ve seen with cancer for example.
The immune system’s failure to destroy senescent cells leads to excessive inflammation and the development of metabolic inflammatory conditions such as obesity and type 2 diabetes, arthritis, osteoporosis and atherosclerosis (stiffening of the arteries), as well as in neurodegenerative diseases such as Parkinson’s or Alzheimer’s.
Upregulating autophagy then is super important if we want to slow aging.
Calorie restriction and fasting can do that as do some senolytics like spermidine, fisetin, quercetin and others.
But fasting can be difficult and is contraindicated for many people so turning to senolytics can be an easier way of clearing out these damaged senescent cells.
I liken the whole process to bringing in the groceries to your house and then preparing meals. That's like bringing in the nutrients etc and the autophagy piece is when you take out the garbage. If you just bought in food to your kitchen everyday, cooked and ate but didn't clear out the garbage you would have a stinking messy, rotten kitchen pretty soon that wouldn't allow you to operate in efficiently anymore.
That's what this whole autophagy/senescent cell story is all about.
So what exactly does Spermdine do in the body.
Well, without going deep into the biochemistry (I will add links to studies below for those science geeks who want to dive deep), and with the understanding that much much more research is needed to elucidate all it's mechanisms of action, here an overview.
Spermidine is known to regulate various cellular processes including:
DNA Stability
Cellular growth
Cellular differentiation
- Apoptosis (Which is cellular death as a normal part of growth and development in organisms)
Research in treating aged mice with spermidine found that it led to a decrease in arterial stiffening, which is an aging-related condition correlated to the deterioration of autophagy
What can Spermidine help with?
It can help with the immune system
Heart Health
Aging and longevity
Prevent Neurodegeneration
In the brain Spermidine reduces inflammation factors, increases the formation of memory cells, and triggers the preventive removal of toxic protein aggregates in cells, which may be responsible for neurodegeneration, the cause of dementia and diseases like Parkinson’s and Alzheimer’s.
Spermidine supports cellular respiration and increases mitochondria (the little powerhouses that produce the energy or ATP in your cells)
In animal studies spermidine extends lifespan by up to 25% (not to mention improving healthspan)
A recent study showed (https://www.nature.com/articles/s43587-022-00322-9)
that Spermidine works on multiple different pathways.
I am a fan of many longevity supplements, and often cycle through various ingredients at different times for different effects but Spermidine is one of the very few I constantly take. Sometimes I do higher doses (4 caps) and others lower doses (2 caps)
If you want to try Spermidine check out ours in our store
References:
Fontana, L. The scientific basis of caloric restriction leading to longer life. Curr. Opin. Gastroenterol. 25, 144–150 (2009).
Lee, C. & Longo, V. Dietary restriction with and without caloric restriction for healthy aging. F1000Res 5, F1000 (2016).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell. Biol. https://doi.org/10.1038/s41580-021-00411-4 (2021).
Longo, V. D., Di Tano, M., Mattson, M. P. & Guidi, N. Intermittent and periodic fasting, longevity and disease. Nat. Aging 1, 47–59 (2021).
Hofer, S. J., Carmona-Gutierrez, D., Mueller, M. I. & Madeo, F. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol. Med. https://doi.org/10.15252/emmm.202114418 (2021).
Hofer, S. J., Davinelli, S., Bergmann, M., Scapagnini, G. & Madeo, F. Caloric restriction mimetics in nutrition and clinical trials. Front. Nutr. 8, 717343 (2021).
Ingram, D. K. & Roth, G. S. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp. Gerontol. 46, 148–154 (2011).
Ingram, D. K. et al. Calorie restriction mimetics: an emerging research field. Aging Cell 5, 97–108 (2006).
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J. & Kroemer, G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29, 592–610 (2019).
Madeo, F., Eisenberg, T., Pietrocola, F. & Kroemer, G. Spermidine in health and disease. Science 359, eaan2788 (2018).
Pegg, A. E. Mammalian polyamine metabolism and function. IUBMB Life 61, 880–894 (2009).
Teixeira, D., Santaolaria, M. L., Meneu, V. & Alonso, E. Dietary arginine slightly and variably affects tissue polyamine levels in male swiss albino mice. J. Nutr. 132, 3715–3720 (2002).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Gupta, V. K. et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 16, 1453–1460 (2013).
Nishimura, K., Shiina, R., Kashiwagi, K. & Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 139, 81–90 (2006).
Jänne, J., Raina, A. & Siimes, M. Spermidine and spermine in rat tissues at different ages. Acta Physiol. Scand. 62, 352–358 (1964).
Ferioli, M. E. & Comolli, R. Changes of liver and kidney polyamine levels during ageing. Exp. Gerontol. 10, 13–15 (1975).
Das, R. & Kanungo, M. S. Activity and modulation of ornithine decarboxylase and concentrations of polyamines in various tissues of rats as a function of age. Exp. Gerontol. 17, 95–103 (1982).
Ferioli, M. E., Sessa, A., Tunici, P., Pinotti, O. & Perin, A. Aging and polyamine acetylation in rat kidney. Biochim. Biophys. Acta 1317, 15–18 (1996).
Liu, P., Gupta, N., Jing, Y. & Zhang, H. Age-related changes in polyamines in memory-associated brain structures in rats. Neuroscience 155, 789–796 (2008).
Zwighaft, Z. et al. Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metab. 22, 874–885 (2015).
Pekar, T. et al. Spermidine in dementia: relation to age and memory performance. Wien. Klin. Wochenschr. 132, 42–46 (2020).
Pucciarelli, S. et al. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 15, 590–595 (2012).
Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation and autophagy to reverse B cell senescence. Mol. Cell 76, 110–125 (2019).
Alsaleh, G. et al. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. eLife 9, e57950 (2020).
Elworthy, P. & Hitchcock, E. Polyamine levels in red blood cells from patient groups of different sex and age. Biochim Biophys. Acta 993, 212–216 (1989).
Soda, K., Uemura, T., Sanayama, H., Igarashi, K. & Fukui, T. Polyamine-rich diet elevates blood spermine levels and inhibits pro-inflammatory status: an interventional study. Med. Sci. 9, 22 (2021).
Uemura, T., Akasaka, Y. & Ikegaya, H. Correlation of polyamines, acrolein-conjugated lysine and polyamine metabolic enzyme levels with age in human liver. Heliyon 6, e05031 (2020).
Morrison, L. D., Becker, L., Ang, L. C. & Kish, S. J. Polyamines in human brain: regional distribution and influence of aging. J. Neurochemistry 65, 636–642 (1995).
Igarashi, K. & Kashiwagi, K. Use of polyamine metabolites as markers for stroke and renal failure. in Polyamines (eds. A. E. Pegg & R. A. Casero) vol. 720, 395–408 (Humana Press, 2011).
Cheng, M.-L. et al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J. Am. Coll. Cardiol. 65, 1509–1520 (2015).
Pan, X. et al. Alzheimer’s disease-like pathology has transient effects on the brain and blood metabolome. Neurobiol. Aging 38, 151–163 (2016).
Graham, S. F. et al. Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS ONE 10, e0119452 (2015).
Schuller, A. P., Wu, C. C., Dever, T. E., Buskirk, A. R. & Green, R. eIF5A functions globally in translation elongation and termination. Mol. Cell 66, 194–205 (2017).
Lubas, M. et al. eIF5A is required for autophagy by mediating ATG3 translation. EMBO Rep. 19, e46072 (2018).
Frankel, L. B. EIF5A mediates autophagy via translation of ATG3. Autophagy 14, 1288–1289 (2018).
Liang, Y. et al. eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction. Cell Rep. 35, 108941 (2021).
Schroeder, S. et al. Dietary spermidine improves cognitive function. Cell Rep. 35, 108985 (2021).
Beyer, H. S., Ellefson, M., Sherman, R. & Zieve, L. Aging alters ornithine decarboxylase and decreases polyamines in regenerating rat liver but putrescine replacement has no effect. J. Lab. Clin. Med. 119, 38–47 (1992).
Wang, W. et al. Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool. Oxid. Med. Cell. Longev. 2014, 457429 (2014).
Wang, J. et al. Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging 12, 650–671 (2020).
Yang, D., Oike, H., Furuse, M. & Yasuo, S. Spermidine resets circadian clock phase in NIH3T3 cells. Biomed. Res 42, 221–227 (2021).
Madeo, F. et al. Nutritional aspects of spermidine. Annu. Rev. Nutr. 40, 135–159 (2020).
Yin, Z., Pascual, C. & Klionsky, D. J. Autophagy: machinery and regulation. Micro. Cell 3, 588–596 (2016).
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634–650 (2021).
Pyo, J.-O. et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 4, 2300 (2013).
Bjedov, I. et al. Fine-tuning autophagy maximises lifespan and is associated with changes in mitochondrial gene expression in Drosophila. PLoS Genet 16, e1009083 (2020).
Schinaman, J. M., Rana, A., Ja, W. W., Clark, R. I. & Walker, D. W. Rapamycin modulates tissue aging and lifespan independently of the gut microbiota in Drosophila. Sci. Rep. 9, 7824 (2019).